
How battery works?
How do batteries work?
A simplified walkthrough of how a battery works
There are many battery types in the market, such as Alkaline battery, lithium battery, Ni-mh battery and so on.
Batteries power many things used in our daily life, and they vary in size and composition depending on what they power. A TV remote is powered by a single AA battery, while an electric car is powered by a huge connection of lithium batteries. But even though they are different battery types, the basics are the same.
A battery basically consists of three elements:
A positive electrode (+) which is called the anode. This is what you see as a “+” sign on the battery.
A negative electrode (-) which is called the cathode. This is what you see as a “–“ sign on the battery.
Electrolyte which separates the cathode and the anode
The goal for the battery is to deliver electricity, which is an energy produced by the flow of electrons. So how does the battery produce a flow of electrons?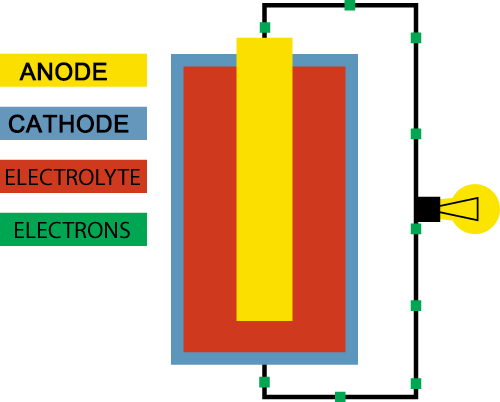
Inside the battery chemical reactions are going on. Both the anode and the cathode react with the electrolyte, which separates the two. In the chemical reaction between the anode and the electrolyte electrons are created, and they will accumulate at the anode.
Since electrons naturally repel each other they will look for a way to escape to an environment with less electrons, and luckily the chemical reaction between the cathode and the electrolyte enables the cathode to accept electrons.
However, the only way for the electrons to travel from the anode to the cathode is if we create a path between the two. This could be by connecting the positive and the negative ends of the battery with a wire. In this circuit we can add a device, like a lightbulb, and the flow of electrons from the anode to the cathode will power the lightbulb. This is actually how a very, very basic flashlight works.
As the battery is being used the chemistry inside the battery changes and the resistance becomes greater until the flow of electrons completely stops. This is how a battery runs out of power.
With a rechargeable battery on the other hand, the whole process can go backwards with the addition of an external electricity source.
What happens is that the positive ions go back from the electrolyte to the anode, and the electrons also travels back from the cathode to the anode. This process primes the battery to do it all over again, or in other words; it is recharged.
